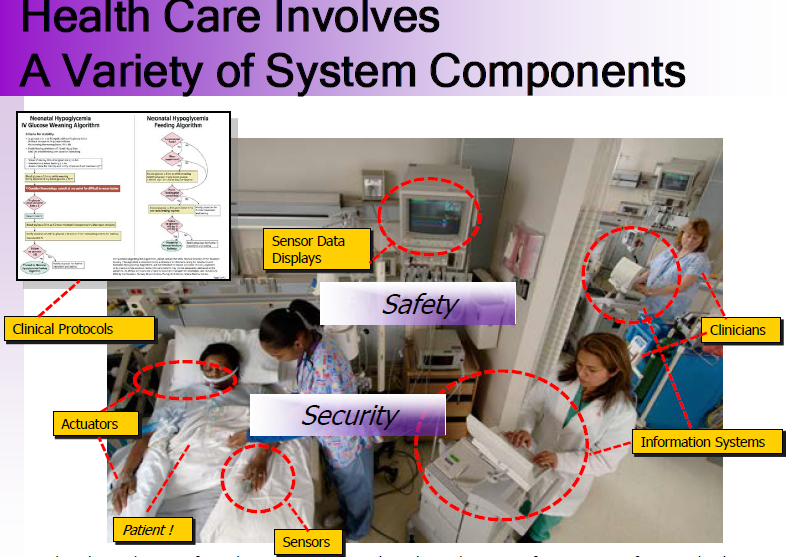
MD Regulations address the direct implications of interoperability in the safety issues.
Now a days the AAMI/UL 2800 standard (association of advanced Medical Interface / Underwriters Laboratory) defines safety and related specifications of Medical Device (MD) interface to be labeled or declared as INTEROPERABLE MEDICAL DEVICE. The standard specify MD interface characteristics to operate in safety conditions and focus on the mitigation of risk associated with interoperability within the ICE (Integrated Clinical Environment) and IS (Interoperable Scenario).
It is complementary of the IEEE 11073 interface (plug & play standard) and particularly in mobile environments with the IEEE-11073- PHD (personal health device) . It is also complementary with the HL7 providing the type of interoperability that improve care delivery, optimize workflow, reduce ambiguity and enhance knowledge transfer and that IHE (Integrated healthcare enterprises) initiative have specify.
In the PoC (point of care) integration, there are several clinical scenarios that the ASTM F29.21 committee has outline: • Data structure ; • Data interchange ; • Semantic content ; • Security ; • Pharmacy and medicines business; • Devices ; • Business requirements for EHRs ; • SDO harmonization
As well as DICOM ISO 12052:2006, or requirements stablished by the Continua Health Alliance, IEC-TC62 joined with the ISO-TC 215.
If these are the things healthcare workers have to use in the XXI century, there will be always the same question: Why they are not aware? Why they do not study those items among others in the carrier in order to carry out an adequate telemedicine and mHealth?.
Many posts in our blog stress the need of a structured training on those items as essential.